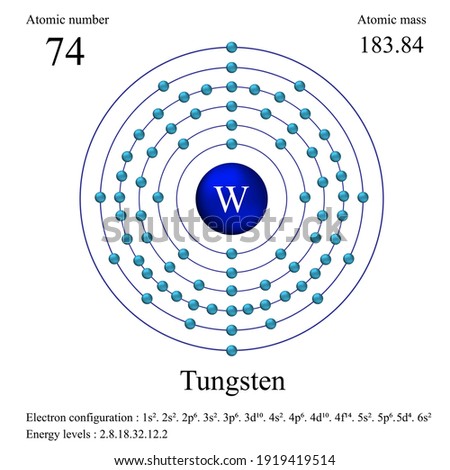
Atomic Number of Tungsten. Atomic Number of Tungsten is 74. Chemical symbol for Tungsten is W. Number of protons in Tungsten is 74. Atomic weight of Tungsten is 183.84 u or g/mol. Melting point of Tungsten is 3407 °C and its the boiling point is 5927 °C.
Tungsten, like all the elements having a higher atomic number than iron (Z>26), cannot be formed by nuclear fusion processes in stars, as is the case for those elements with a lower atomic number, but originates only by neutron or proton absorption of already existing bigger nuclei. These capture processes with extremely high fluxes of neutrons and protons occur exclusively in massive stars (>8 times the solar mass) during the end of their life cycle. Massive stars end in a supernova explosion whereby certain amounts of their mass are distributed to the surrounding space, including also the tungsten atoms formed.
The name Wolfram is closely related to today’s important tungsten mineral wolframite. In the Middle Ages (16th century) tin miners in the Saxony-Bohemian Erzgebirge in Germany reported about a mineral which often accompanied tin ore (tinstone). From experience, it was known that the presence of this mineral reduced the tin yield during smelting. Foam appeared on the surface of the tin melt and a heavy deposit formed in the smelting stove, which retained the valuable tin. 'It tears away the tin and devours it like a wolf devours a sheep', a contemporary wrote in the symbolic language of those times. The miners gave this annoying ore German nicknames like 'wolffram', 'wolform', 'wolfrumb' and 'wolffshar' (because of its black colour and hairy appearance). Georgius Agricola was the first to report about this new fossil (Spuma Lupi) in his book “De Natura Fossilium”, published in 1546.
The name Tungsten came from the other important tungsten ore, which is now called scheelite. In 1750, this heavy mineral was discovered in the Bispberg´s iron mine in the Swedish province Dalecarlia. The first person who mentioned the mineral was Axel Frederik Cronstedt in 1757, who called it Tungsten {composed of the two Swedish words tung (heavy) and sten (stone)} due to its density close to 6.
In 1781, the outstanding Swedish chemist Carl Wilhelm Scheele published the results of his experiments on the mineral tungsten in Kongl. Vetenskaps- Academiens Nya Handlingar, with the title: “The Constituents of Tungsten”. In this work he demonstrated that the mineral contains lime and a still unknown acid, which he called tungstic acid. Torbern Bergman, professor in Uppsala, suggested preparing the corresponding metal by charcoal reduction of the obtained acid.
183.950928 (3) 30.64 (2) 0. 185.954357 (4) 28.43 (19) 0. Isotope abundances of tungsten. In the above, the most intense ion is set to 100% since this corresponds best. Atomic mass is an absolute mass, relative isotopic mass is a number without proportions and without units. To measure the number of atoms in a sample you will figure out how many moles the sample element contains. A mole is the choice of unit chemists. It’s equal to Avogadro’s number (6.02 X 1023) of atoms. The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Atomic mass of tungsten = total number of protons and neutrons in a nucleus of tungsten. Therefore the neutrons of tungsten = Atomic mass of tungsten- protons of tungsten Atomic mass of tungsten = 183.84 Tungsten protons = 74.
Tungsten Atomic Mass
In 1781/1782, the Spanish nobleman, Juan José de D´Elhuyar studied metallurgical chemistry with Prof Bergman and gathered information about the work on the mineral tungsten. Back to Spain in 1783, Juan José analyzed a wolfram species from a tin mine in Zinnwald/Saxony, and showed it to be an iron and manganese salt of a new acid. He also concluded that wolfram contained the same acid as Scheele had gained from tungsten. He then reduced the oxide to the new metal by heating it with charcoal, as had been recommended by his teacher Bergman.

His discovery, jointly with his brother Fausto Jermin, was published in 1783 by the Royal Society of Friends of the Country in the City of Victoria (“Analysis quimico del volfram, y examen de un Nuevo metal, que entra en su composition por D Juan Joséf y Don Fausto de Luyart de la Real Sociedad Bascongada”). The new metal was named VOLFRAM after the mineral used for analysis.
Thereafter, an increasing number of scientists explored the new chemical element and its compounds. However, the price for the metal was still very high and the time was not yet ripe for promising applications.
In 1847, a patent was granted to the engineer Robert Oxland. This included the preparation of sodium tungstate, formation of tungstic acid, and the reduction to the metallic form by oil, tar or charcoal. The work constituted an important step in modern tungsten chemistry, and opened the way to industrialisation.

Tungsten Element Atomic Model
First, tungsten-containing steels were patented in 1858, leading to the first self-hardening steels in 1868. High speed steels with tungsten additions up to 20% were first exhibited at the World Exhibition in Paris in 1900, and revolutionized engineering practice in the early 20th century. Such steels (Taylor- and White) are still used today in practically every machine shop of the world.
The first tungsten light bulbs were patented in 1904, and rapidly replaced the less efficient carbon filament lamps on the lighting market. Since then, tungsten filaments have illuminated the world and have revolutionized artificial lighting in general.
To produce drawing dies with diamond-like hardness but improved toughness was the driving force for the development of cemented carbides in the 1920s. At this time, no one, even the most optimistic, could imagine the enormous breakthrough for this material in the tooling industry. After WW2, a huge market opened in the growing economies and cemented carbides contributed as tool materials and construction parts for their industrial development.
In 1944, K C Li, President of Wah Chang Corporation in the US, published a picture in the Engineering & Mining Journal entitled: “40 Years Growth of the Tungsten Tree (1904 – 1944)” illustrating the fast development of the various tungsten applications in the field of metallurgy and chemistry.
To compare the evolution of a technology with the growth of a tree was a unique idea which has since been developed and expanded from 1850. This 150 years’ time span reveals a fascinating picture of scientific and technological evolution.
The tree has grown to reach today a peculiar form, which is dominated by an increasingly thick bole (cemented carbides) with two main branches (steel and mill products). The chemicals branch seems somehow atrophied but still has a large number of small leaves. Due to the unique properties of tungsten, it can be assumed that in future a steady further growth will occur, given the appropriate market opportunities.
For the full story on the History of Wolfram and Tungsten, click to read the article in Newsletters June and December 2005. Further information is given in the Tungsten Brochure (2009).
The Trewhiddle Tungsten Bloom
The ITIA does not often receive calls from TV producers but there was excitement over the discovery of a lump of metal found in the West of England, after an article was published in “Materials World” in February 2004.
Tungsten Atomic Mass Number

There was speculation that this lump was tungsten metal, pre-dating its presumed discovery in 1783, and a further twist to the story suggests the involvement of that scientist, man of letters, bankrupt and thief, Rudolph Erich Raspe (1737-1794), the author of “The Travels of Baron Munchausen”.
Tungsten Atomic Mass In Kg
For the story and more information, click to view Newsletter June 2005.

Comments are closed.